t-cell dynamics matter
Lessons from the First Treg Immunotherapy Trial
Regulatory T cells (Tregs) are suppressor cells by definition. Usual T cells induce immune response when they recognise their target. However, Tregs can suppress immune response. Thus the current theory is that Tregs are a special type of T cells, functioning to maintain immune system balance and prevent autoimmune diseases, where the body attacks its own cells.
However, these T cells aren’t as unique as people believe. This is illustrated in the catastrophic clinical trial for the world-first clinical trial, TGN1412 (Bending & Ono, 2018).
The Clinical Trial
The significance of understanding T cells’ dynamics in vivo is illuminated by the clinical trial of TGN1412 in 2006. TGN1412, a superagonistic anti-CD28 antibody intended to suppress autoimmune reactions, instead caused severe inflammation known as a “cytokine storm” in volunteers.
Turning Point
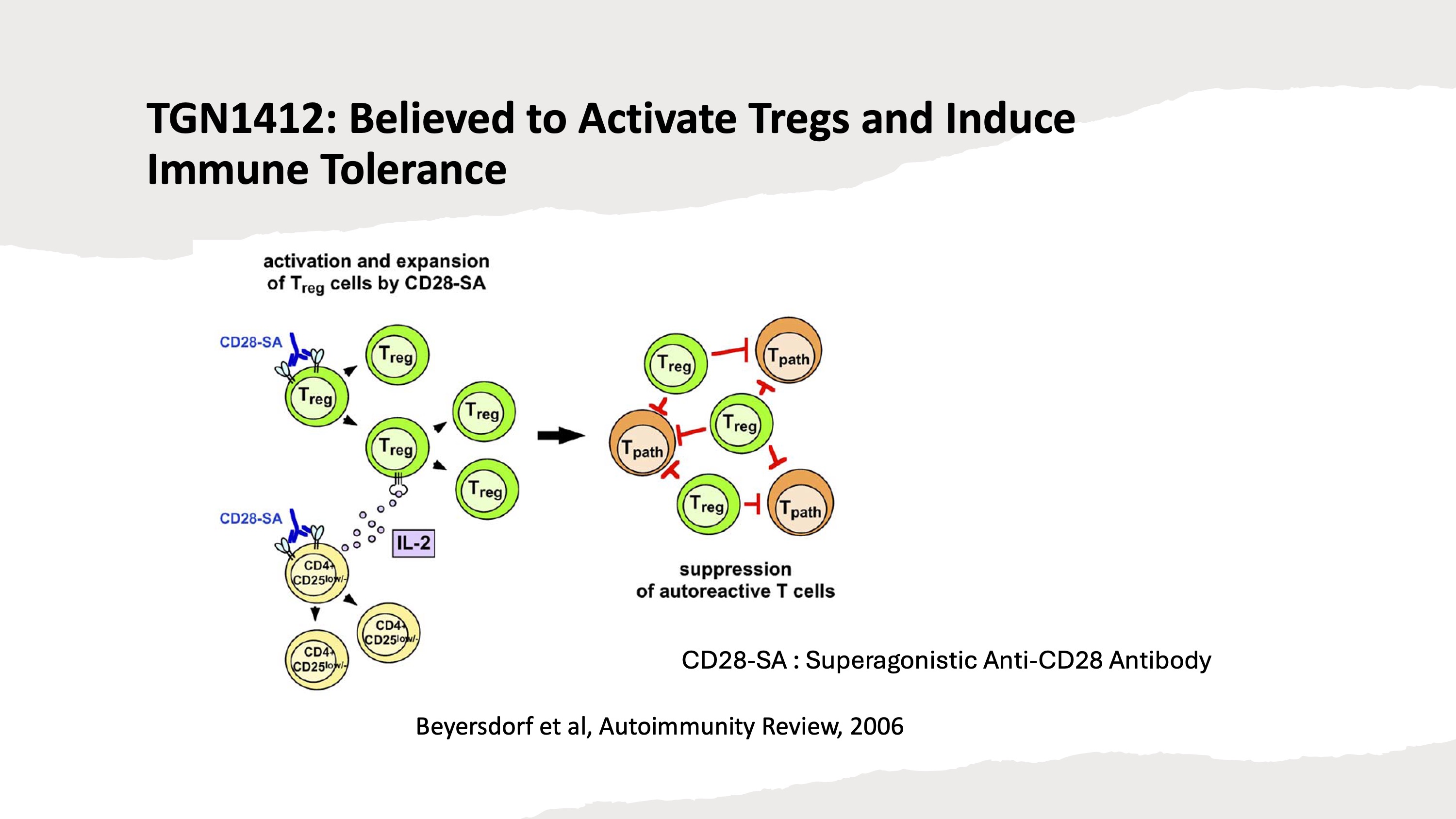
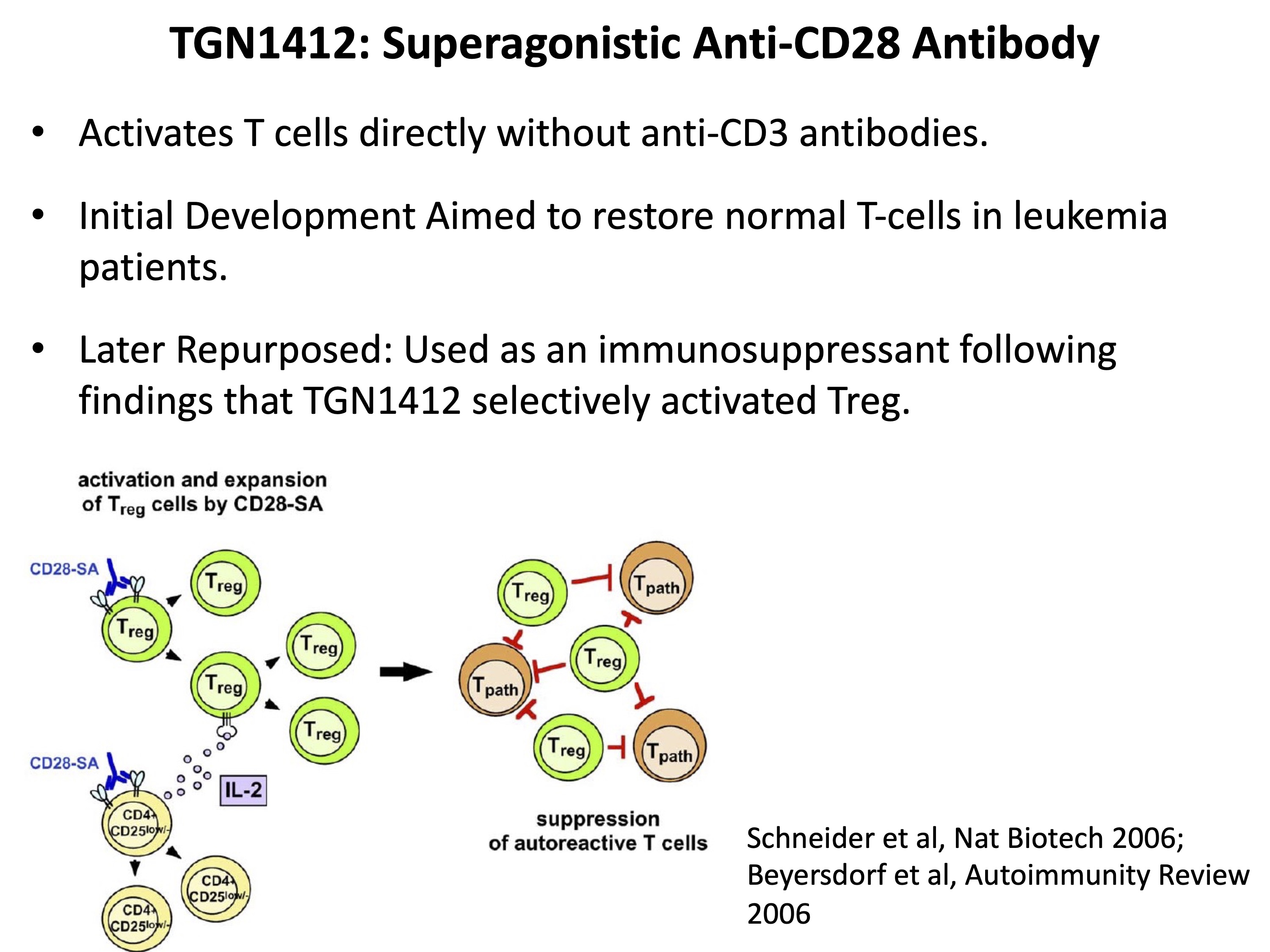
TGN1412 is a superagonistic anti-CD28 antibody designed to activate T cells directly, eliminating the need for anti-CD3 antibodies. Initially, it was developed with the goal of activating and restoring the T-cell repertoire in leukaemia patients. However, following promising results from animal studies, TGN1412 was later repurposed as an immunosuppressant. These studies showed that the anti-CD28 stimulation selectively activated regulatory T cells (Tregs) and effectively suppressed immune response.
Investigations and Reflections
Investigations into the incident concluded that it was an unfortunate and unforeseeable event due to the different expression dynamics of CD28 between humans and primates, which were used in the pre-clinical stage of the drug development.
However, as discussed in Vitetta and Ghetie (2006), Tregs and non-Tregs may not represent strictly separate lineages. Therefore, the assumption of specifically activating only a minor population may have been inappropriate.
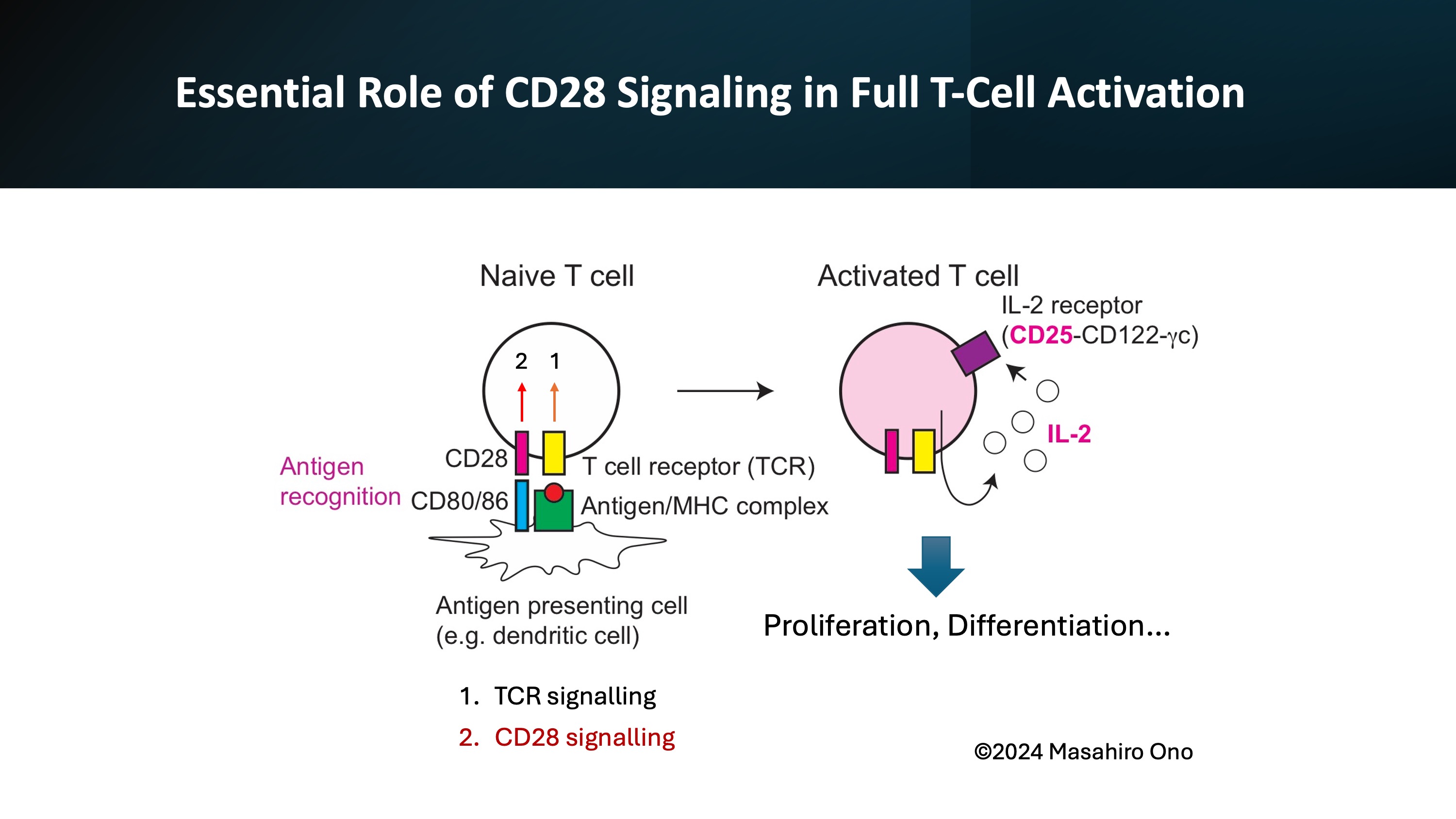
It is worth remembering that the first lecture in T cell biology always begins with T cell receptor signaling and CD28 signaling. Without CD28 signaling, T cells cannot be fully activated, as CD28 signaling is a strong activating signal for all T cells.

This disaster underscored the importance of understanding the roles and activation mechanisms of T cells in general, not just focusing on Treg cells alone.
Foxp3 and the Lieage Perspective
Asano et al set the stage for the lineage perspective of Treg by highlighting CD25+CD4+ T cells as a “naturally differentiated, unique T cell subset originating in the thymus.” My other article showed that this perspective was not supported by reproducible evidence at the time.
However, the subsequent development in immunology—the discovery of Foxp3—fit well with the stage set by Asano et al. Foxp3, as a transcription factor specifically expressed by CD25+CD4+ T cells, was defined as the lineage-determinant factor of Tregs.
It is true that Foxp3 is key in controlling the development and function of suppressive T cells. However, by defining Foxp3 as the lineage factor, we overlook an essential aspect of T cells – their dynamic nature and changing properties.
Treg Plasticity or Changing T cells?
Recent studies have highlighted the plasticity of Tregs—that is, their ability to change under certain conditions. For example, during inflammation, Tregs can lose their Foxp3 expression and transform into effector T cells, which actively respond to pathogens. This plasticity is crucial because it demonstrates that Tregs can adapt based on the body’s needs, potentially shifting roles from regulators to attackers.
However, by categorizing these changes as Treg plasticity, studies remain trapped and constrained by the lineage perspective. Indeed, many researchers and pharmaceutical companies are pursuing the development of a method to produce ‘stable’ Tregs.
What if Foxp3 is inherently a dynamic molecule? Is it an effective approach to pursue the stable Treg as a holy grail?
From Stability To Dynamics
Revealing the principles of Foxp3 as a dynamic mechanism could significantly improve our ability to control the T cell system. However, this cannot be achieved if studies remain constrained by the lineage perspective that originated with Asano et al.
Studying the dynamic changes in Tregs during immune responses is crucial for a deeper understanding of immune regulation. Innovative tools like the Tocky system, which tracks cell kinetics and activity, are enabling researchers to explore how Tregs and their Foxp3 expression change in real-time. These insights are vital for developing effective immunotherapies.
Understanding the dynamics of Foxp3 and Treg activity provides a window into the sophisticated mechanisms of immune regulation. This knowledge is not only fundamental to the field of immunology but also crucial for developing new treatments for immune-related diseases.
Investigating Foxp3 as a dynamic molecule and the dynamics of T cells in vivo within a broader context will be a fundamental approach not only for understanding T cell biology but also for developing future immunotherapies.
The contents of this article are discussed in detail in my paper published in 2019 and my blog article “Decoding Foxp3 – From Treg Dogma to T-cell Dynamics”.

References
2018
- From stability to dynamics: understanding molecular mechanisms of regulatory T cells through Foxp3 transcriptional dynamicsClinical and Experimental Immunology, Sep 2018
Enjoy Reading This Article?
Here are some more articles you might like to read next: