The Dogma of Treg and The True Origins of Tocky
Introduction
Regulatory T cells (Tregs) are often described as key players in controlling the immune system and maintaining immune tolerance. However, today I aim to shed light on some serious issues with the fundamental evidence supporting the role of Tregs. In this blog, I will discuss the cardinal issues in Treg biology that have permeated communities and hindered advancements in our understanding of T cell regulation. We will explore what can truly be trusted and what cannot. This reflection and my persistent efforts to overcome these challenges have shaped my research journey over the past two decades.
Discovery of Immunosuppressive T Cells
In immunology research in the 1970s and 1980s, it became clear that T cells not only induce immune responses by using cytokines and surface molecules, but also play a suppressive role. Central to this was the day 3 thymectomy experiment by Yasuaki Nishizuka and colleagues.
Right after a mouse is born, its blood and spleen have almost no T cells. Thus, if the thymus is removed from a newborn mouse, the mouse develops immunodeficiency and dies. This phenomenon was first discovered by Jacques Miller (Jacques Miller) and led to the revelation that the thymus is crucial in T cell development.
However, if the thymus is removed on the third day after birth, the mouse does not become immunodeficient—instead, it develops autoimmune disease. It was Yasuaki Nishizuka and his colleagues (see footnote) who made this discovery (Nishizuka & Sakakura, 1969).
Determined to unravel the mystery of autoimmune diseases induced by thymectomy, Nishizuka continued his research. Over a decade of detailed studies led him to make an interesting prediction: that “suppressor T cells” do not appear in the periphery before day three after birth (Taguchi et al., 1980). In other words, if such special cells were found, Nishizuka believed they would be these elusive “suppressor T cells.”
However, despite Nishizuka’s work and predictions, the mechanism by which day 3 thymectomy causes autoimmune disease remained a mystery, continuing to captivate many researchers. Nishizuka passed away before resolving this mystery. And to this day, the mystery remains unsolved.
From Nishizuka’s Day 3 Thymectomy Autoimmune Disease Research to Shimon Sakaguchi’s Regulatory T Cells
In 1977, Shimon Sakaguchi came to train under Nishizuka at the Aichi Cancer Center, where day 3 thymectomy-induced autoimmunity was being studied. During this time, Sakaguchi learned Nishizuka’s theory and neonatal thymectomy techniques (Sakaguchi et al., 1982).
After leaving Nishizuka’s lab, Sakaguchi went to the United States for further research, then returned to Japan as an independent investigator studying autoimmune diseases induced by thymectomy, among other topics.
In 1995, Sakaguchi and colleagues demonstrated the “suppressive activity” of CD25⁺CD4⁺ T cells using an adoptive transfer experiment (Sakaguchi et al., 1995). However, because CD25 was then known to be expressed on essentially any activated T cell, the distinction from typical activated T cells was obscure, and their work initially did not gain much attention.
To break this stalemate, In 1996, Sakaguchi published a paper showing that CD25⁺CD4⁺ T cells supposedly do not appear in the periphery until after day three of life (Asano et al., 1996).
Even though the Asano et al. paper does not mention Nishizuka by name, it effectively “proved” that the CD25⁺CD4⁺ T cells were exactly the “suppressor T cells” Nishizuka had predicted.
Of particular note, Dr. Ethan Shevach enthusiastically supported the Asano et al. findings (for a time), thereby amplifying research into regulatory T cells. As a result, regulatory T cells - now identified as CD25⁺CD4⁺ T cells and established as a unique lineage of T-cells produced in the thymus - have gained the fame, making breakthroughs.
The Study That Formed Today’s Treg Concept Is Not Supported By Data
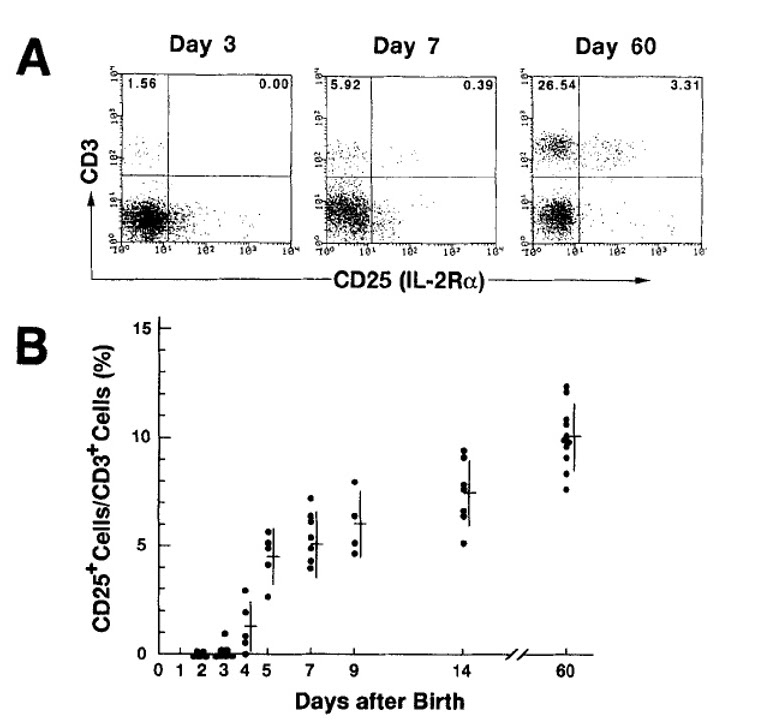
Asano et al. reported that CD25⁺ T cells do not appear until after the third day of a mouse’s life, showing a rapid increase in CD25⁺ T cells only after day four.
My Own PhD Study
From 2002 to 2003, during my PhD work, I discovered that a substantial number of CD25⁺ T cells are already present in the spleen and thymus from day one after birth. This directly contradicted Asano et al.’s report, and the results were consistent across multiple repetitions.
In 2003, in my second year of the doctoral program, I retested the Asano et al. findings. The results differed significantly from theirs: there were plenty of CD25⁺ T cells in the spleen and thymus of newborn mice from day one. Subsequent checks on days two, three, seven, one week, and two weeks after birth consistently showed no major differences.
These results clearly contradict the main claim of Asano et al.’s landmark 1996 paper on Tregs, and repeated attempts only reinforced my findings. At that point, I became convinced that something fundamental was amiss in the field of regulatory T cell research.
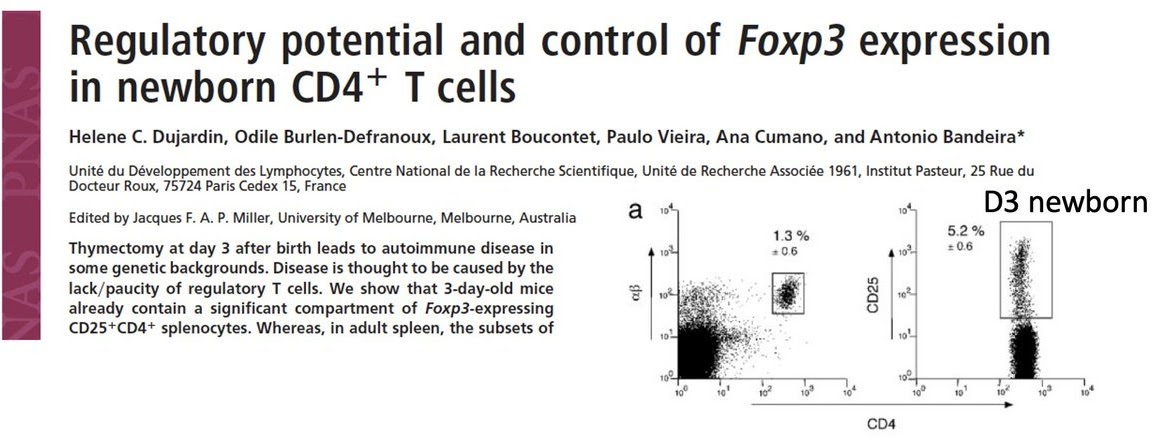
Moreover, I was not the only one to make this finding. Data reported by the Bandeira research group (Dujardin et al., PNAS, 2004) were consistent with my observations.
A Community Who Does Not Dare To Care
I thought there was no need for me to publish my results right away because the French group led by Bandeira published identical data around the same time (Dujardin et al., PNAS, 2004). Their paper also demonstrated that neonatal mouse spleens indeed harbor sufficient numbers of CD25⁺ T cells. This evidence scientifically invalidated the core claims of the Asano paper and raised strong calls for reevaluating the regulatory T cell theory.
Later, the same findings as those of Bandeira’s group were confirmed using Foxp3 reporter mice by the research team led by Kenneth Tung, a renowned specialist in thymectomy-induced autoimmunity (Samy et al., 2008).
Yet, the findings by Bandeira’s and Tung’s groups were completely ignored by the immunology community. Many textbooks and review articles continued to feature Asano et al.’s discovery while neglecting the contrary evidence about the dynamics of neonatal regulatory T cells.
In 2015, I published a paper scrutinizing the Asano et al. findings, clarifying that the cardinal data was not reproducible and rebutted by multiple studies (Ono & Tanaka, 2016).
However, even today — see for example Svoboda, Nature, 2022 — the outdated Asano et al. paper is still cited when discussing regulatory T cells. This perpetuates the unfounded claim that Tregs do not appear before three days after birth, echoing like a dogma chanted by a medieval priesthood.
Why Asano et al. Matters Today
While the concept of thymus-derived Treg, which stems from Asano et al. in 1996, has been widely accepted, my reproducibility experiments, along with those of other groups, have raised serious questions about the validity of the prevailing understanding of Tregs.
Simply speaking, if the fundamental evidence that has established a field is not reproducible, shouldn’t we go back to the root of the problem more carefully?
Many people may immediately rebut my idea. We know Tregs exist. Tens of thousands of papers have been published showing the importance of Tregs in various medical and biological fields, all supported by these papers, maybe except for the defect in Asano et al.
However, the problem of Asano et al is a central issue in T cell biology and immunology - because the current understanding of all genes and cells related to Treg has been distorted by the theory established by Asano et al - i.e. There is a developmentally distinct population of T cells that are dedicated to controlling immunological tolerance.
Some may argue that this idea—the unique lineage of Treg—was established not by Asano et al. but by discoveries related to Foxp3.
No, it was not. The understanding of Foxp3 would have been completely different—and more productive—had the paper by Asano et al. not existed and not misled the immunology community.
Thus, I have a fundmental question here - How Immunology would look should the concept of Treg not have been introduced?
What Cannot Be Measured Should Not Be Trusted
Let us discern between what is definitively known and what remains uncertain in the immunological disciplines that deal with Treg.
Definitively Measurable and Established
- Expression of Foxp3: This is quantifiable and can be consistently measured.
- Expression of Key Markers (e.g., CD25): These markers are quantifiable through various assays.
- CD4 T Cell Activities (e.g., proliferation, cytokine production): These activities are directly measurable using established methods.
Ambiguously Defined and Unmeasurable
- Regulatory T Cells (Tregs) Dedicated to Controlling Immunological Tolerance: How do we measure whether a Treg cell is actively controlling immunity? This remains unquantifiable.
- Suppression of T Cell Activities by Tregs: How can we determine if a T cell’s function is being suppressed by a Treg? This interaction is not directly measurable.
- Thymic Tregs (tTregs) and Peripheral Tregs (pTregs): Since their proposal in 2013, no reliable method has emerged to distinguish whether a Treg is thymic or peripheral. Thus, identifying tTregs vs. pTregs remains impractical and unmeasurable.
The Problem
Treg Immunology relies excessively on variables that are not directly measurable, leading to speculative conclusions rather than grounded science.
The Solution
We must adhere strictly to data—what can be empirically measured—to reestablish immunology as a rigorous science. This approach, which we term “Data-Oriented Immunology,” prioritizes tangible evidence over theoretical constructs.
My Own Journey Toward Data-Driven Immunology and the Breakthrough via Canonical Correspondence Analysis
Now I’d like to go back to my own research journey in the past 17 years.
Reflecting on the last 17 years, I’ve identified pivotal issues in 21st-century immunology—a realization that first struck me in 2008. This epiphany compelled me to deviate from conventional narrative-driven approaches, firmly guiding me towards data-driven science rather than pursuing story-driven articles in prestigious journals.
Even after publishing my own paper in Nature in 2007, I could not subscribe to the purported value of top-ranked journals. Instead, I believe that what truly matters is the data and its appropriate analysis, not the crafting of narratives.
Thus, I decided to leave Japan and dedicate three years, supported by the Human Frontier Science Program Fellowship, to exploring and developing a new methodology in immunological genome analysis between 2009 and 2013.
Fortuitously, the late Professor Robin Callard at University College London (UCL), who hosted my HFSP fellowship, was exceptionally supportive and nurturing. He granted me complete freedom during these three years, allowing me to fully dedicate myself to research.
The breakthrough came when I developed Canonical Correspondence Analysis (CCA) for genomic data, establishing a multidimensional method to quantitatively analyze T cell phenotypes. Conveniently, the British Library was near UCL’s Institute for Child Health, allowing me access to a wealth of statistical books and enabling me to learn various multidimensional methods.
CCA emerged as the ideal methodology for quantitatively analyzing the phenotypes of uncharacterized cells by comparing them to prototype cell phenotypes in relation to gene expression profile gradients.
To my ecologist friends reading this—this approach is very similar to ter Braak’s analysis of fish species distribution in ocean locations relative to ion concentration, isn’t it?
Development of Tocky in a Quiet Laboratory
Yes, being in a quiet place is crucial for INTP individuals like me to fully utilize their creativity.
Building on these computational skills, in 2013, I initiated a technological innovation to create a novel experimental methodology. This involves the use of a Fluorescent Timer protein that spontaneously changes its emission spectrum at known kinetics, enabling the analysis of the temporal dynamics of T cell activities.
No Discussion, No Paper Reading, and No Google
Fortunately, in 2013, I was awarded a significant fellowship, the BBSRC David Phillips Fellowship, to launch my own laboratory and create something completely new. My research proposal was to synthesize methods in experimental and computational analysis.
I decided to dedicate the first month of the fellowship to designing and refining the new approach that can reveal the fate of individual T cells in vivo—without discussing with other people, without reading PubMed papers, and without Googling at all.
Should such a method be developed, we would be able to understand whether and how individual T cells change their phenotype—’lineage’, which I believe is a likely event in vivo.
Path to Tocky is via CCA
Using CCA, I identified Nr4a3 as a gene downstream of T cell receptor (TCR) signaling. With the Nr4a3 Fluorescent Timer reporter developed, we should be able to quantitatively measure the temporal dynamics of TCR signaling activities within individual T cells.
Additionally, a Foxp3 Fluorescent Timer reporter should address the fundamental questions regarding Treg biology stemming from the non-reproducible data in Asano et al.
Again, fortunately, I had full proficiency in molecular biology to develop Transgenic reporter constructs efficiently. I acquired the skills to use Bacterial Artificial Chromosome in Kyoto, Japan, from 2006 to 2009, but none of my developed knockout animals was published as they were part of an industrial grant for developing a novel seed that becomes a medicine. However, in 2013 in London, I was able to fully utilize these skills to quickly develop two Timer reporter lines—now known as Nr4a3-Tocky and Foxp3-Tocky.
To make the molecular project as effective as possible, I chose not to recruit any postdocs. Instead, I served as the postdoc for the Principal Investigator myself.
This was how 2013 was spent—in one of the most productive ways in my research life.
If you are a new Principal Investigator and have never worked on your own, you should not miss the great opportunity to use your own time exclusively for your creativity. Such a time might never come again in academic life, at least in the UK!
Tocky As Integration of Experimental and Computational Methods
After my collaborating embryologists shipped back the new Tocky mice in 2014—more than one year after the start of my fellowship—I finally started to recruit a technician. She was a hard worker, and together, we began to analyze the brand-new Tocky mice.
And I realized a remarkable thing: experiments alone are not enough. I needed to develop a dedicated computational methodology to fully analyze the flow cytometric data that Tocky mice generate.
Eventually, the journey toward developing this computational methodology took even longer than expected. Now, in 2025 — 12 years after I began developing Tocky — I finally feel comfortable enough to declare that the computational methodology part has been fully developed and is ready to be launched.
(To be continued.)
If you found this blog post interesting, please see our paper (Ono & Tanaka, 2016) in which we analyze and discuss these Treg issues in greater detail.
References
-
J F Miller and D Osoba, “Current concepts of the immunological function of the thymus.” Physiological Reviews 1967 47:3, 437-520. https://pubmed.ncbi.nlm.nih.gov/4864541/
-
Nishizuka Y, Sakakura T. “Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice.” Science. 1969 Nov 7;166(3906):753-5. doi:10.1126/science.166.3906.753
-
Taguchi O, Nishizuka Y, Sakakura T, Kojima A. “Autoimmune oophoritis in thymectomized mice: detection of circulating antibodies against oocytes.” Clin Exp Immunol. 1980 Jun;40(3):540-53. PMID: 6998618
References
2016
- Controversies concerning thymus‐derived regulatory T cells: fundamental issues and a new perspectiveImmunology and cell biology, May 2016A landmark opinion piece challenging the reproducibility of a foundational Treg experiment, while introducing a groundbreaking dynamic view on Foxp3-mediated T cell regulation.
Enjoy Reading This Article?
Here are some more articles you might like to read next: